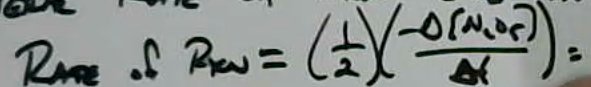Skip to main content
Chapter 14
Chapter 14: Chemical Kinetics
Rates of reaction and the particulate nature of matter
- Kinetics:
- Rates of reaction (speed)
- The sequential steps of a reaction
- Affect the rates?
- Concentration of the reactants
- The more stuff that is present, the more collisions will occur and the rate of reaction will increase
- Temperature of reaction
- As the temperature increases, the rates also increase
- Structure and orientation of particles
- B-A + C → A-C + B
- A-B + C -/->
Rates in a chemical reaction
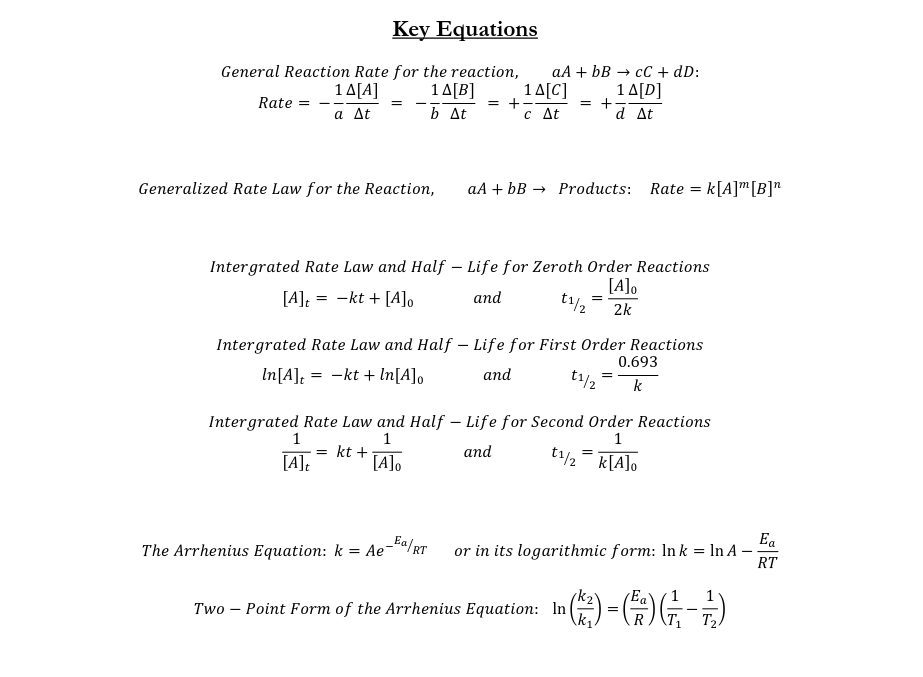
- Rate = $\frac{\text{Concentration Change}}{\text{Time Change}}$
- 2 N2O5 → 4NO2 + O2
- Rate of formation for NO2: $\frac{\Delta [NO_2]}{\Delta t} = 3.7\times 10^{-5} M s^{-1}$
- Rate for formation for O2: $9.00\times 10 ^{-6} M s^{-1}$
- Rates must be positive
- General rate of reaction
- Instantaneous rate: rate of reaction at a single point in time
- It is the slope tangent to the curve
The Rate Law
- How the reaction proceeds over the entirety of the reaction
- aA + bB → products
- Rate Law: $\text{Rate} = k[A]^m[B]^n$
- These exponents tell us how sensitive the reaction is to changes in concentration
- These exponents must be experimentally determined
- This is because there are sequential steps for the reaction
- The larger the exponent, the more sensitive it is to changes in concentration
- 3 common reaction orders that we can think about
- We mean exponents by orders (m/n = 0, 1, 2)
- Technically they can go higher or negative, but that is beyond the scope of General Chemistry II
- If n = 0, the change in concentration has no effect on the rate
- If n = 1, the rate is directly proportional to the concentration
- If n = 2, the rate is proportional to the square of the concentration
- Overall reaction order: sum exponents
- $\text{Rate} = k[A]^2[B]^1, \text{rate} = 3$
Determining the Order of a Reaction
- Initial rates: start of reaction
- Change concentration and see the effect on the rate
The Integrated Rate Laws
First Order Integrated Rate Law
- $\text{Rate} = k[A]^1 = \frac{-\Delta [A]}{\Delta t}$
- $ln[A]_t = -kt + ln[A]_0$
Second Order Integrated Rate Law
- $\text{Rate} = k[A]^2 = \frac{-\Delta [A]}{\Delta t}$
- $\frac{1}{[A]_t} = +kt + \frac{1}{[A]_0}$
Zeroth Order Integrated Rate Law
- $\text{Rate} = k[A]^0 = \frac{-\Delta [A]}{\Delta t}$
- $[A]_t = -kt + [A]_0$
The Half-Life of the Reaction
- The time needed for the concentration to be one half of its initial value
- First order: $ln[A]_t = -kt + ln[A]_0$
- $ln(\frac{[A]_t}{[A]_0}) = -kt$
- $[A]_t = \frac{1}{2}[A]_0$
- $ln(\frac{\frac{1}{2}[A]_t}{[A]_0}) = -kt$
- $-ln(2) = -kt_{\frac{1}{2}}$
- $t_{\frac{1}{2}} = \frac{ln(2)}{k}$
- For the first order reaction, $t_{\frac{1}{2}}$ has no concentration dependence
- Second order: $t_{\frac{1}{2}} = \frac{1}{k[A]_0}$
- Zeroth Order: $t_{\frac{1}{2}} = \frac{[A]_0}{2k}$
The Effect of Temperature on Reaction Rates
- The Arrhenius Equation
- $k=Ae^{\frac{-Ea}{RT}}$
- A is the frequency factor
- -Ea is the activation energy
- R is the gas law constant
- T is the temperature in kelvin
- Activation energy
- The amount of energy required to make the reaction go from products to reactants
- Change in energy is just final minus initial
- Frequency factor ($A$)
- Number of times R approaches Ea barrier per unit of time
- Exponential factor ($\frac{-Ea}{RT}$)
- Fraction of molecules with energy to get over the barrier
- Two-point form of the Arrhenius equation
- $ln(A) = ln(k_1) + \frac{Ea}{R}(\frac{1}{T_1}) = ln(k_2) + \frac{Ea}{R}(\frac{1}{T_2})$
- The collision model (A)
- Need 2 properly oriented molecules with sufficient energy to get over the barrier
- Orientation factor
- The reactants need to be oriented properly
- Collision frequency (z)
- Collision rate = $z[A][B]$
- Reaction rate = orientation factor * collision rate * exponential factor
Reaction Mechanisms
- Series of molecular steps to get from reactants to products
- Elementary steps
- Individual molecular event
- Reaction intermediate
- Made in one step and consumer in another
- Never seen in an equation
- Rates laws for elementary steps: molecularity
- Unimolecular: one reactant (Rate = $k[O_3]$)
- Bimolecular: two reactants (Rate = $k[O_3][O]$)
- Termolecular: three reactants (exceedingly rare) (Rate = $k[O]^2[M]$)
- Assumptions
- Elementary reactions can be reversible
- Intermediates cannot be in the overall reaction or the overall reaction law
- Rate determining step is the slowest step
- Conditions for a plausible mechanism
- The elementary steps must sum to the overall reaction
- The rate law from the mechanism must match the experimentally determined rate law
- Molecularity must make sense
- Look out for this
- Only have termolecular if there is NO other option
- Slow first step, fast second step
- Rate is based on the first step
- The overall rate law is based on the slowest step